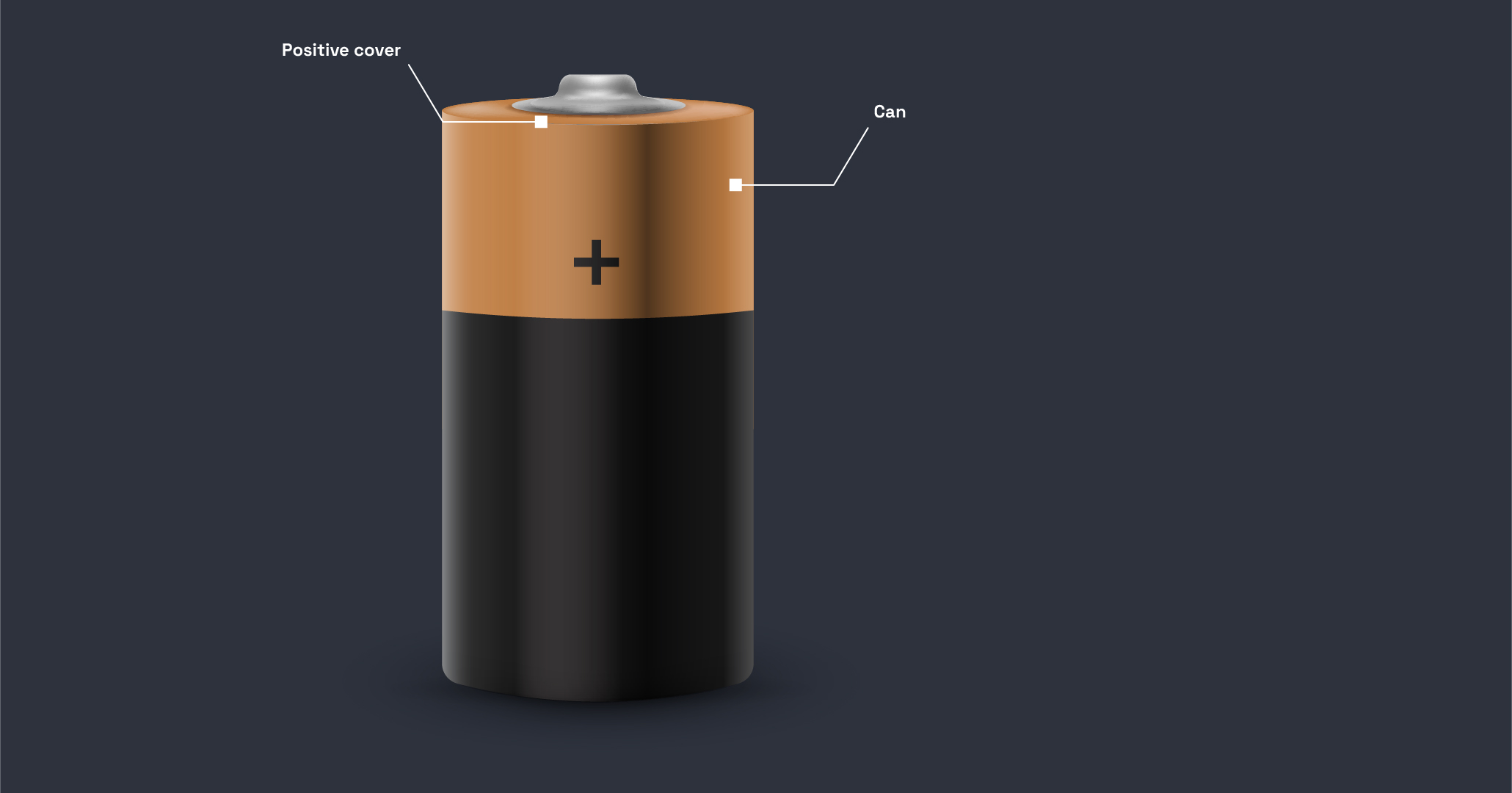
What does a lithium-ion battery do?
Cells in a battery store, transform, and conduct energy.
A lithium-ion battery and circuit transform electrochemical energy into electrical energy and transmit that energy as a current to a device, which is known in a circuit as a “load.”
The opposite reaction also occurs: When a battery is being charged, the movement of electrons and ions transforms electrical energy into electrochemical energy. The battery stores this electrochemical energy.
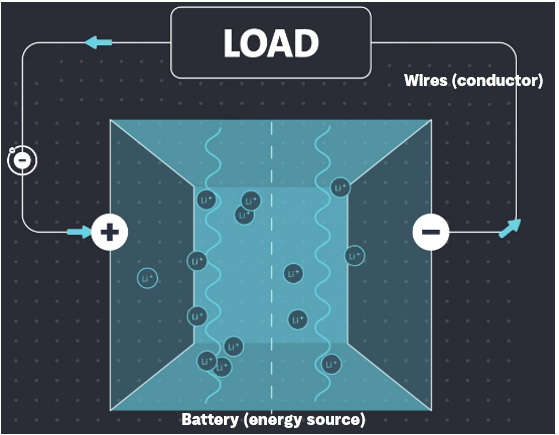
Circuits
Batteries work within a circuit, which includes an energy source, a conductor, and a load (device receiving energy).
A short circuit occurs when electrical current flows on an unintended path where it meets too little resistance. A short circuit can happen inside or outside of a cell. The result is that too much electrical energy flows through this new path.
A short circuit within a lithium-ion battery can lead to thermal runaway.
Interested in learning more about short circuits? Check out this video.