The Science of

There’s been a fire. Investigate the burn patterns and figure out where the fire started and how.

The Science of

Fire safety is a complex problem without a single answer. Learn how to engineer and design fire-safe spaces.

The Science of

Safe and sustainable cities will depend on lithium-ion batteries to power our modern lives. But what are the costs?

The Science of

Lithium-ion batteries in your favorite devices make our daily lives possible, but they also come with risks. Join the search for safer solutions.

touch_app Interactive
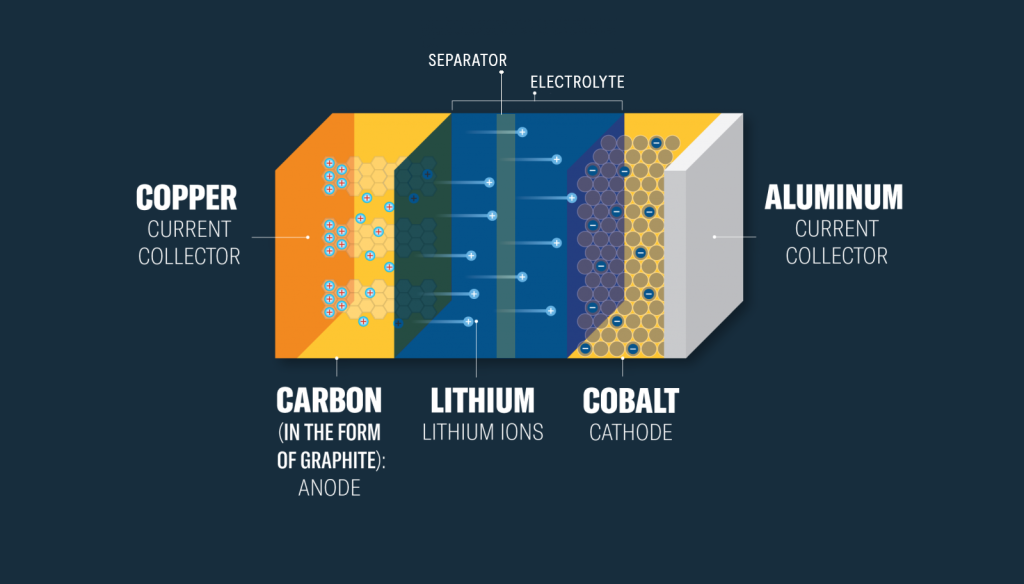
Lithium-Ion Battery Cross-section
Find this resource
© 2026 Underwriters Laboratories Inc. All rights reserved.